by Harveen Kaur
Abstract
The presence and rapid spread of the novel infectious disease COVID-19, caused by the respiratory pathogen/coronavirus SARS-CoV-2, has encompassed the world as a global pandemic. COVID-19 spreads contagiously through air and droplets of bodily fluids, such as saliva, and has an array of symptoms associated with its contraction and spread, leading to approximately 364 cumulative coronavirus-related hospitalizations per 100,000 individuals only in the United States during early 2021. Additionally, COVID-19 cases are often more severe in individuals that have pre-existing medical conditions, with sickle cell disease being at the top of the list. Sickle cell disease, also referred to as sickle cell anemia, is an inherited blood disorder that abnormally distorts the structure and function of hemoglobin in the body leading to oxygen deficiency. This medical condition has been associated with increasing the risk of developing a severe case of COVID-19, but a definite connection has not yet been confirmed that categorizes the risk factor for various age groups. Studies published in September 2020 report that an international registry, called SECURE-SCD, has been created to track patients suffering from both conditions and their respective health outcomes. By using the data from various documented studies, this article hypothesizes that there is an important connection between sickle cell anemia and COVID-19 that leads to enhanced symptoms and overall higher fatality in patients suffering from the two. Hence, the primary purpose is to dissect and evaluate the specific linkage between the SARS-CoV-2 coronavirus and sickle cell anemia including the risk factors for both medical conditions experienced simultaneously and based on key demographic categories. Establishing the connection between sickle cell disease and COVID-19 is significant to better understand the implications of the virus for those with pre-existing conditions and potentially adjust treatment protocols that are currently being discovered.
Introduction
Infectious diseases have proven to be the ‘silent killer’ of the world throughout history. Ranging from the Black Death bubonic plague, the Spanish flu, and the swine-origin influenza A (H1N1) virus, the slow but steady upward trend of infectious disease occurrence enforces the fact that a human-dominated world can never thrive with imbalances in nature [1]. Many of these infectious diseases are zoonoses, diseases in animals that are transmitted to human beings, while others are classified as viruses transmitted by human beings to other human beings [2]. Virulent microorganisms have been present among humankind for thousands of years, but it is only within the past couple of centuries that they have caused a huge leap in mortality. The most recent example of a globally destructive pathogen is the SARS-CoV-2 coronavirus, which causes the respiratory syndrome [2].
COVID-19, a respiratory illness that the world has been facing as a pandemic for over one year, is a contagious infectious disease stemming from the SARS-CoV-2 respiratory pathogen with the most common symptoms as fatigue, cough, and fever [3]. According to the CDC, the virus primarily spreads through respiratory droplets that arise through coughing and/or sneezing [3]. However, recent studies and speculation have stated that transmission may be airborne due to the presence of SARS-CoV-2 viral RNA in air samples within droplet nuclei that stay infectious if left in the air over long distances and large amounts of time [4]. Currently, there is no definitive cure for COVID-19, but many countries including the United States have implemented methodologies to track and contain the virus, especially through vaccine development. Specifically, roughly 78 vaccines for COVID-19 are undergoing clinical trials, with seven vaccines (such as those developed by Pfizer-BioNTech and Moderna) already distributed for full use [5]. These highly infected countries have also encouraged individuals to socially distance themselves from others by at least six feet and wear masks, both of which help control daily cases and fatalities from COVID-19 [6]. Despite these preventative measures, the implications of COVID-19 have affected millions of individuals in the United States and on a global scale. Vulnerable populations that already suffer from pre-existing medical conditions are at high risk to develop and contract a severe case of COVID-19. One such diagnosis that has caused an uproar in the United States is sickle cell disease.
Sickle cell disease (SCD), also called sickle cell anemia, consists of a group of inherited disorders that changes the shape of red blood cells from disc-shaped to crescent-shaped when atypical hemoglobin is present [7]. Through this change, red blood cells are more prone to blocking blood flow and do not move easily in the body, which can lead to an onset of chronic conditions such as strokes and other pain crises [7]. With the spread of COVID-19, however, those suffering from SCD have been studied to have a higher rate of contraction, as well as more severe symptoms and outcomes when coupled with the virus [8]. For example, SCD patients with COVID-19 have a higher overall case fatality (10.9% fatality rate) than non-SCD individuals contracting the virus (3.3% fatality rate) [9]. Patients suffering from SCD have cardiopulmonary comorbidities that COVID-19 heightens, but the specific linkage between the two has yet to be closely compared. However, certain unmistakable trends connecting patient’s age, race and severity of SCD symptoms to their COVID-19 prognosis have been observed [8]. This article focuses primarily on the results from various national and international registries that collect information about COVID-19 and SCD by comparing the effects of suffering from both conditions together, based on age groups and other patient demographics. Establishing and characterizing the connection between the two conditions can create a more accurate solution on how to protect oneself from COVID-19 when suffering from SCD.
Materials/Methods
The connection between SCD and COVID-19 transmission and symptoms, when specifically focusing on age and race, can be outlined through the data found in three studies.
The first study, published on the Centers for Disease Control and Prevention, was conducted from March to May 2020 in the United States and was recorded in the Medical College of Wisconsin SECURE-SCD Registry [10]. This particular registry examines COVID-19 cases among those living with SCD in the United States, and the results of the study were limited to a two-month time period in which such patients were analyzed and documented. The study primarily focused on the average age of these patients, the number of SCD-related complications these patients have had in the past, and the resulting intensity of their current COVID-19 symptoms [10].
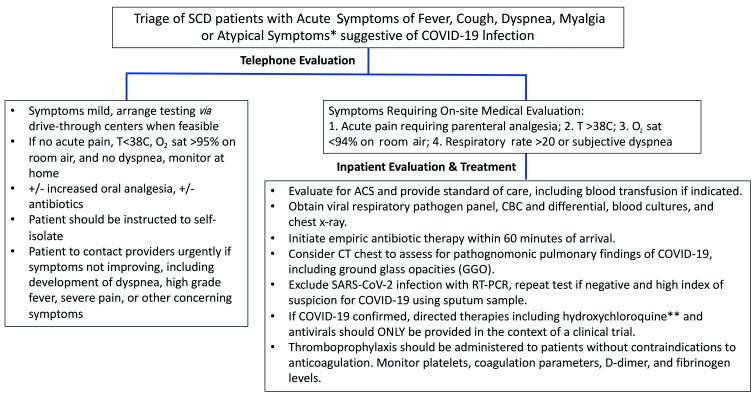
Another study, published in the journal Haematologica, referenced 10 patients in the UK who had contracted SARS-CoV-2, and all 10 patients had hemoglobin SS disease, which is the most common and most severe type of SCD [11]. The severity of this particular kind of SCD also means that these patients experience the worst symptoms at the highest rates for the condition [12]. With the most recent documentation being published in November 2020, the recovery rates of these patients were analyzed. The figure below from the same study highlighted the steps of evaluation conducted for the patients with SCD who had symptoms that aligned with potentially contracting COVID-19 as well as further standards of treatment and evaluation based on individual patient status [11]. The diagram shows the process of triage for patients with SCD who also contracted COVID-19 based on symptoms and patient accessibility [11].
Figure 1 Determining the appropriate medical response for SCD patients with COVID-19, ranging from patient instructions, types of medical evaluation, and potential therapies [11].
A third study, presented at the American Society of Hematology, was led by Dr. Lana Mucalo from the Medical College of Wisconsin and required the examination of 370 COVID-19 cases in patients already suffering from SCD from an international sickle cell registry [13]. This particular study compared the data found from the patients listed in the international registry to patients suffering from both conditions in the general Black population, keeping race as the measured demographic independent variable [13].
Results
The results of the first study, documented by the Medical College of Wisconsin SECURE-SCD Registry in between March 20, 2020 and May 21, 2020, showed that roughly 178 individuals who had SCD — and who were not simply carriers of the sickle cell trait — also contracted COVID-19 during this time. [10] Out of these 178 individuals, 7% (13 individuals) died upon contracting COVID-19 and being hospitalized, and 11% (19 individuals) were admitted to the intensive care unit while suffering from both conditions simultaneously [10]. More than half of the total individuals had suffered an SCD-related complication in the past that led to prior hospitalizations, and the average age of the 178 individuals at the time was roughly 28.6 years [10]. On the contrary, individuals with COVID-19 that don’t suffer from SCD have a death rate of less than 1% for those 20—54 years of age in early 2020, which is dramatically less than rates analyzed for patients with both SCD and COVID-19 [14].
From the 10 patients in the UK documented in Haematologica, a 54-year-old patient died who suffered from delayed hemolytic transfusion reactions and also had high levels of CRP (C-reactive protein), a marker of negative prognostics for patients suffering from COVID-19 [11]. From all 10 patients, two underwent hydroxyurea therapy, seven had regular blood transfusions, and a total of five patients were managed remotely through telephone contact for their treatment [11]. Medical professionals and experts believed that suffering from the SARS-CoV-2 infection along with SCD would lead to acute chest syndrome (ACS). However, in the 10 patients, only one of them suffered from related respiratory complications; this was the same patient who passed away during clinical monitoring [11].
In the final study, the Black population was examined since, especially in the United States, individuals with African-American descent are statistically more likely to have both the sickle cell trait as well as the disease [13]. Additionally, completely separate from SCD, Black Americans are nearly four times more likely to be hospitalized due to COVID-19 as the virus has progressed globally [13]. Using race as the measured variable, this study concluded that those with SCD are roughly 6.2 times more likely to suffer a fatal complication from COVID-19 when compared to the general African-American population in the United States [13]. While the fatality rate based on ages 18-34 and 35-50 for the general Black population is less than 1% for all Black people in both age groups, those with SCD had death rates of 2.6% and approximately 12%, respectively, for the same age groups [13]. Additionally, a significantly larger amount of COVID-19 hospitalizations for Black individuals with SCD occurred among the younger age group, which correlates with larger death rates as age decreases [15].
Discussion
With the knowledge of all three pieces of data, these results illustrate that there is a correlation regarding age and race when examining the compounding effects of SCD and COVID-19. When examining the whole percentage of individuals who were hospitalized and severely affected by suffering from both conditions, the data from the SECURE-SED Registry suggests that such a connection exists. Additionally, data from the registry includes reports of 7% fatalities and 11% admittance to the ICU over 60 days for individuals with both conditions, which is heavily alarming. Even in the UK, the data suggests that there is an evident conjunction between the two conditions, despite the study being conducted with a small sample size. If 1 out of 10 individuals died, a 10% mortality rate from the combined detrimental effects of both conditions is just as concerning, if not more, than the first study. Lastly, the final study examined the effects of racial background on contracting COVID-19 while having SCD, and a significant relationship between the two is evident. In fact, having SCD, particularly for Black Americans, dramatically increases the fatality rate of these individuals from COVID-19 (by up to 11%). Based on these aforementioned studies, strong evidence exists to suggest that SCD and COVID-19 do have detrimental implications for individuals on a global level.
Conclusion
After understanding the symptoms of patients already suffering from SCD, adding the effects of COVID-19 can exponentially deteriorate a patient’s health. Through the three different pieces of data, there is a noticeable connection between incidence of SCD and severity of symptoms of COVID-19, the most recent and prevalent globally-spread virus. These connections can be analyzed on the basis of age as well as race, thereby indicating that the hypothesis for a linkage between the two conditions is well-supported. Since COVID-19 treatments and vaccinations are still being actively manufactured, knowing its added implications for those with SCD can be significant when determining a proper healing regimen for the virus. Additionally, understanding the damage done by both ailments combined can help reduce their high mortality rate, thereby creating more successful outcomes for patients for both diseases individually and together.
Works Cited
[1] Bean, Mackenzie. Becker’s Hospital Review. 2020.
[2] Tabish, Syed Amin. International Journal of Health Sciences. 2009, 3(2) V-VIII.
[3] Coronavirus Disease 2019 [Online]. 2021. https://www.cdc.gov/dotw/covid-19/index.html (accessed Mar. 17, 2021).
[4] Liu, Jiaye. Emerg Infectious Dis. 2020, 26(6), 1320-1323.
[5] Zimmer, Carl. The New York Times. 2021.
[6] Thu, Tran P. B. Elsevier Pub Health Emerg Cond. 2020, 742.
[7] National Heart, Lung and Blood Institute. US Dept. of Health & Human Services.
[8] Shet, Arun. American Society of Hematology. 2021.
[9] Minniti, Caterina P. Blood Advances. 2021, 5(1), 207-215.
[10] Panepinto, Julie A. Centers for Disease Control and Prevention. 2020.
[11] Menapace, Laurel A. Haematologica. 2020, 105 (11), 2501-2504.
[12] Rogers, Graham. Healthline. 2019.
[13] Thompson, Dennis. Medical Xpress. 2020
[14] Razzaghi, Hilda. Centers for Disease Control and Prevention. 2020, 69(12), 343-346.
[15] Mucalo, Lana. American Society of Hematology. 2020.