by Akshay Sethi
Introduction:
Escherichia coli (E. coli) is a rod-like anaerobic bacterium that infects the gastrointestinal tract of humans (Fig. 1).
Fig 1: (E. coli bacterial cells)
Most E. coli are harmless, but there are pathogenic strains such as Shiga toxin-producing E. Coli (STEC) that can cause diarrhea, abdominal cramping, and/or acute renal failure. [1] E. coli is found to colonize the human body hours after childbirth and does not pose harm unless the gastrointestinal barrier is broken or the host is immunocompromised. The microorganism is a threat to humans. Gram-negative bacteria possess cell envelopes and an outer membrane that can protect the inner membrane and its organelles from antimicrobial enzymes and antibiotics (Fig. 2). [2]
Fig 2: (Gram-negative bacteria have more components, including an outer membrane, to protect it)
The combination of a low concentration of E. coli needed to infect the host and its resistance to antibiotics makes the microorganism dangerous. [3]. E. coli has a core genome, or series of DNA strands that are common in all strains, but the bacteria also has a non-shared gene pool that differentiates each strain from anotherThere are at least six types of deadly pathotypes of E. coli., all of which cause enteric disease, or diseases relating to the gastrointestinal tract, and potentially lead to long-term injury.
E. coli is not highly transmissible, as the risks come from contaminated food such as undercooked meat, unpasteurized milk, dirty water, or contaminated stool. [4] Therefore, the best prevention for the risk of infection is cleanliness such as washing one’s hands, as an individual must ingest the pathogen to contract it. [4]
Novel therapries and treatments for E. coli have shown limited success. [8] Much of the successful experimentation has come from the process of injecting fluid with secretion inhibitors to stop production of key components of the bacterium. The main system for infecting host cells, T3SS, is the main target of secretion inhibitors and has been the focus of modern research on E. coli. [10]
Discussion:
Harms in E. coli:
E. coli varies in its threat to humans based on the amount of Shiga toxins it produces. [3] Shiga toxins act as ribotoxins that inhibit protein synthesis, leading to altered gene expression or even apoptosis. This would lead to bloody diarrhea, or worse, the Hemolytic Uremic Syndrome (HUS). [5] HUS is one of the more dangerous outcomes of contracting harmful E. coli, where infected people suffer red blood infection and potential total kidney failure. [5] Global estimates project about 10% of STEC infections lead to HUS. HUS is the most common cause of acute renal failure in children, one of the two high-risk groups to contracting an E. coli infection. [5] The syndrome can also cause neurological failures in 25% of cases, resulting in symptoms such as seizure, coma, or stroke. [5]
Shiga toxins are found in over 200 strains of E. coli. [7] Shiga toxins found in E. coli are found in the pathogenic serotypes of E. coli as Stx1. [4] A serotype is a specific group of bacteria sharing a common antigen, which is an immunogenic particle that incites an antibody response. Another serotype of E. coli contains Stx2, which is a more potent Shiga toxin and a part of a different antigen serogroup. A serogroup consists of microorganisms differing in their composition of antigens. [4] The Stx toxin is comprised of two key subunits—the A subunit and B subunit. The A subunit’s function is to inhibit protein synthesis by damaging the ribosome in an infected cell. [4] The B subunit binds to the globotriaosylceramide (Gb3), which is found in endothelial cells lining cardiac muscles and blood vessels. [4] Among biological substances, Shiga toxins are amongst the most poisonous and are lethal to various animals in small doses. E. coli carrying the Shiga toxin serotypes have been shown to lead to the worst outcomes of contracting the pathogen. Across different species, the Shiga toxins have been found to cause acute renal damage and in some cases, total renal failure. [7] Within population-based observations, along with experimentation on mice and baboons, Shiga toxins have been linked to HUS. [4]
The pathway for Stx serotypes of E. coli to lead to HUS begins with the consumption of contaminated substances. E. coli rapidly replicates within the host and the Shiga toxins latch onto Gb3 before being enveloped and packaged into a microvesicle. This vesicle is then absorbed into the kidney endothelial cells, where the toxins cause necrosis, eventually leading to acute renal failure (Fig. 3). [6]
Fig 3: (Stx binding to the GB3 on the cell membrane to take over the ribsomal components of the cell)
Besides the kidney, the brain is the second most affected organ in severe E. coli infection. The Shiga toxins attack neurons within the brain and can cause damage or partial failure to the central nervous system. Central nervous system damage can lead to seizure, shock, or paralysis in some cases. [7]
Classifications of Shiga toxins go more in-depth than Stx1/Stx2 to distinguish between the potency of the toxin and the binding receptor. [7] Some types of Stx can cause severe loss of body mass, along with renal failure. [7] One common symptom of E. coli infection shared between most classifications is colon damage. Shiga toxins inhibit the function of the large intestine, which in turn causes severe symptoms such as bloody diarrhea. [7]
In the past couple of decades, Shiga toxin-carrying E. coli has been found in animal-to-animal transmission. [7] In an isolated study, STEC infection was found in zoonotic transmission, and a common source was domestic animals, such as house cats and dogs. Other sources included wild animals, but at similar rates. Most commonly found were feral hogs and hyenas. Scientists have looked to treat E. coli because of the long-lasting impact of the infection on the human body, including high blood pressure, heart disease, or kidney problems. [1]
Secretion Inhibitors:
One of the newly discovered methods in treating E. coli is anti-virulence therapy. This therapy takes advantage of the type III secretion system (T3SS) in E. coli that primarily exists to grow and multiply the bacteria within hosts. [8] The T3SS acts as a needle that will “inject” effector proteins into the epithelial cells of the gastrointestinal tract. Effector proteins are biomolecules that can attach to proteins and affect the production and type of product that the protein will create. These proteins function as ligands as well as secretory proteins, attaching mainly to host cells to infect them. The ligands will lower the activation energy to conduct the process of making bacterial cells, and therefore increase the rate of production. [8] The impetus for targeting T3SS by scientific researchers is that it is the main mechanism that both Enterohemorrhagic E. coli (EHEC) and Enteropathogenic E. coli (EPEC) use to infect and populate the host effectively. EHEC is the strain of E. coli that can cause HUS, while EPEC is less severe in bodily harm but is the leading strain for diarrheal deaths. [8]
The method in which the treatment is proposed to work is unique. Instead of regulating or limiting the growth of new cells, the anti-virulent treatment targets and inhibits a virulence factor. [8] Virulence factors are what cause bacteria to cause disease in eukaryotic, multicellular organisms. [8] These factors will include molecules that will aid bacteria in infecting the host organism’s cells. [12] The factors are categorized as either secretory, membrane associated, or systolic. Systolic factos will lead to the bacteria changing its physiological or physical structure. [12] Secretory virulence factors assist the bacterium in counteracting the host’s immune response by releasing chemicals. [12]
One inhibitor of the T3SS is salicylidene acyl hydrazide, which has been shown to be effective against EPEC, EHEC, and Salmonella. [9] Although effective, the salicylidene acyl hydrazide was discovered to indiscriminately bind to human proteins, which may negatively affect patient metabolism. [10] It is important to note that the effect of salicylidene acyl hydrazide on T3SS is broadly accurate in preventing the secretion system from functioning. [10]
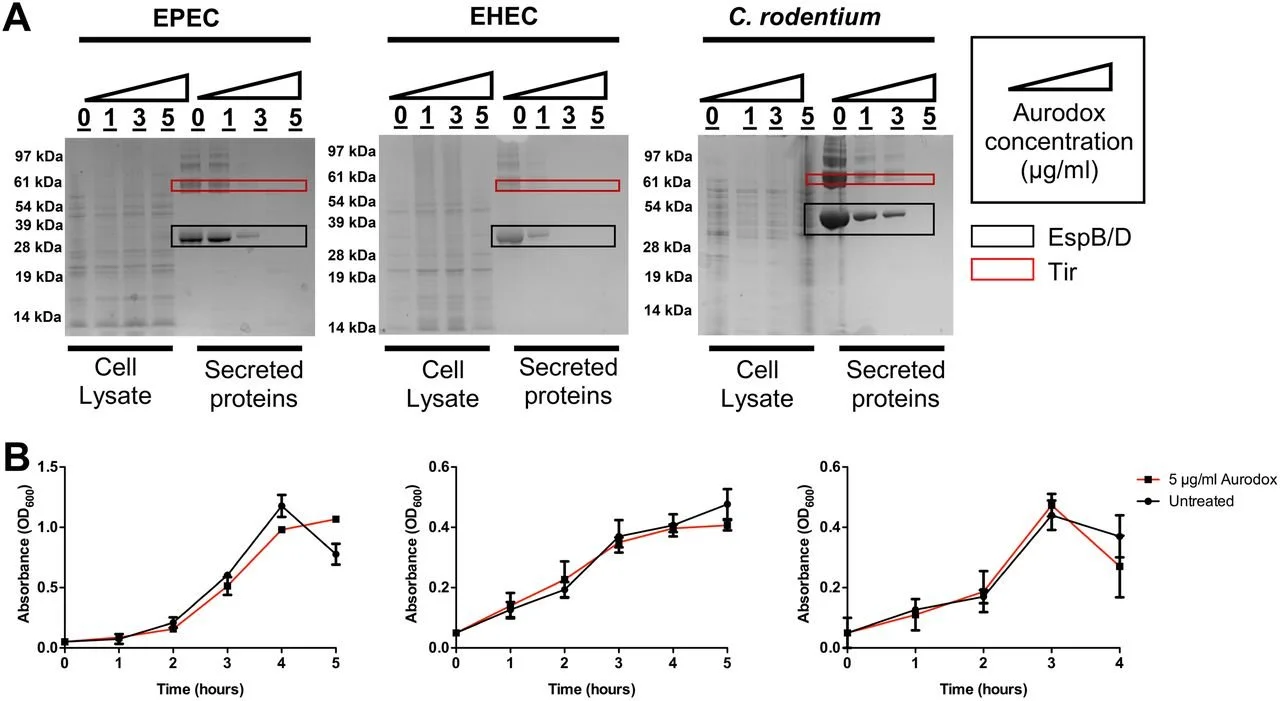
One important study explores the ways a secretion system inhibitor, Aurodox, can affect the T3SS function in different bacteria. [8] Aurodox is the novel system that incorporates salicylidene. [8] Although it does not affect the growth of bacteria, Aurodox has been shown to inhibit the secretion of T3SS. The effectiveness of Aurodox on inhibiting the T3SS system depends on the concentration of the Aurodox injected into the in vivo sample. [8] Researchers have found that in key strains of E. coli, Aurodox did not inhibit growth of the bacterium while inhibiting the mechanism of T3S production (Fig. 4). [8 ]
Fig 4: (Aurodox will inhibit T3S secretion, but not the growth of different bacterial strains)
In the study, researchers found that Aurodox has the function of inhibiting EHEC to infect epithelial cells in infected mice. Although results were inconclusive at the cellular level, Aurodox was shown to reduce colon damage in the mice. [8] The expression of T3SS in various proteins involved in DNA-binding and altering were also shown to have been inhibited by Aurodox.
Conclusion:
These results are promising for the inhibition of T3SS expression in anti-virulent treatment. As noted previously, past antibacterial treatments had induced the SOS response in the EHEC strain of E. coli, which was not the case for the anti-virulent Aurodox. [8] This is significant because the SOS response as a function leads to the release of Shiga toxins. Without the release of the deadly toxins, EHEC infection can be inhibited.
Aurodox as a treatment poses an interesting future for combatting E. coli. While Aurodox can effectively inhibit the secretory function of E. coli through the T3SS system, it does not halt production of the bacterium. This poses a question of whether to accept the cohabitation of EHEC and EPEC strains, among others that are considered harmful in the present. [8] If Aurodox can effectively neutralize the harmful effects of the strains, then there is little reason to eradicate the bacterium from the host.
Drawbacks:
One drawback to current antibiotic treatments which anti-virulent therapy attempts to solve is the SOS response by E. coli. [8] The anti-virulent therapy can cause minor DNA damage, signaling an SOS response. The SOS response will occur after the use of antibiotics and eventually lead to the overproduction of Stx in the intestines. This uptick in Stx production will lead to a higher chance of severe symptoms of the anti-virulence treatment.
Antibiotic drawbacks are more feared by the scientific community because E. coli is the most common pathogen in humans. In a study analyzing 150 different food samples to test the antibiotic sensitivity pattern of the bacteria, the highest percentage of drug-resistant E. coli was found in the most common foods with E. coli present, which included raw meat, eggs, and salad. [11] To combat this, scientists propose that the general population practice good hygiene, and farmers should only use reserve antimicrobial drugs to reduce the possibility for antimicrobial resistance. [11] Anti-virulence treatment does not deal with antimicrobial drugs, and is more likely to reduce unwanted side effects of general treatment of E. coli as compared to other proposed solutions.
[1] E. coli. (2018). Retrieved 20 December 2021, from https://www.who.int/news-room/fact-sheets/detail/e-coli
[2] Kaper, J., Nataro, J., & Mobley, H. (2004). Pathogenic Escherichia coli. Nature Reviews Microbiology, 2(2), 123-140. doi: 10.1038/nrmicro818
[3] Fact Sheet: Escherichia coli - Microbial Identification - MALDI ToF. (2021). Retrieved 20 December 2021, from https://wickhamlabs.co.uk/technical-resource-centre/fact-sheet-escherichia-coli/
[4] Melton-Celsa, A. (2014). Shiga Toxin (Stx) Classification, Structure, and Function. Microbiology Spectrum, 2(4). doi: 10.1128/microbiolspec.ehec-0024-2013
[5] Gram-negative Bacteria Infections in Healthcare Settings | HAI | CDC. (2021). Retrieved 20 December 2021, from https://www.cdc.gov/hai/organisms/gram-negative-bacteria.html
[6] E. coli: What is It, How Does it Cause Infection, Symptoms & Causes. (2021). Retrieved 20 December 2021, from https://my.clevelandclinic.org/health/diseases/16638-e-coli-infection
[7] Kim, J., Lee, M., & Kim, J. (2020). Recent Updates on Outbreaks of Shiga Toxin-Producing Escherichia coli and Its Potential Reservoirs. Frontiers In Cellular And Infection Microbiology, 10. doi: 10.3389/fcimb.2020.00273
[8] https://doi.org/10.1128/IAI.00595-18
[9] Zambelloni R, Marquez R, Roe AJ. 2015. Development of antivirulence compounds: a biochemical review. Chem Biol Drug Des 85:43–55.
[10] Dai Wang, Caroline E. Zetterström, Mads Gabrielsen, Katherine S.H. Beckham, Jai J. Tree, Sarah E. Macdonald, Olwyn Byron, Tim J. Mitchell, David L. Gally, Pawel Herzyk, Arvind Mahajan, Hanna Uvell, Richard Burchmore, Brian O. Smith, Mikael Elofsson, Andrew J. Roe, Identification of Bacterial Target Proteins for the Salicylidene Acylhydrazide Class of Virulence-blocking Compounds*, Journal of Biological Chemistry, Volume 286, Issue 34, 2011, Pages 29922-29931, ISSN 0021-9258, https://doi.org/10.1074/jbc.M111.233858. (https://www.sciencedirect.com/science/article/pii/S0021925819760700)
[11] Rasheed MU, Thajuddin N, Ahamed P, Teklemariam Z, Jamil K. Antimicrobial drug resistance in strains of Escherichia coli isolated from food sources. Rev Inst Med Trop Sao Paulo. 2014;56(4):341-346. doi:10.1590/s0036-46652014000400012
[12] Sharma AK, Dhasmana N, Dubey N, et al. Bacterial Virulence Factors: Secreted for Survival. Indian J Microbiol. 2017;57(1):1-10. doi:10.1007/s12088-016-0625-1